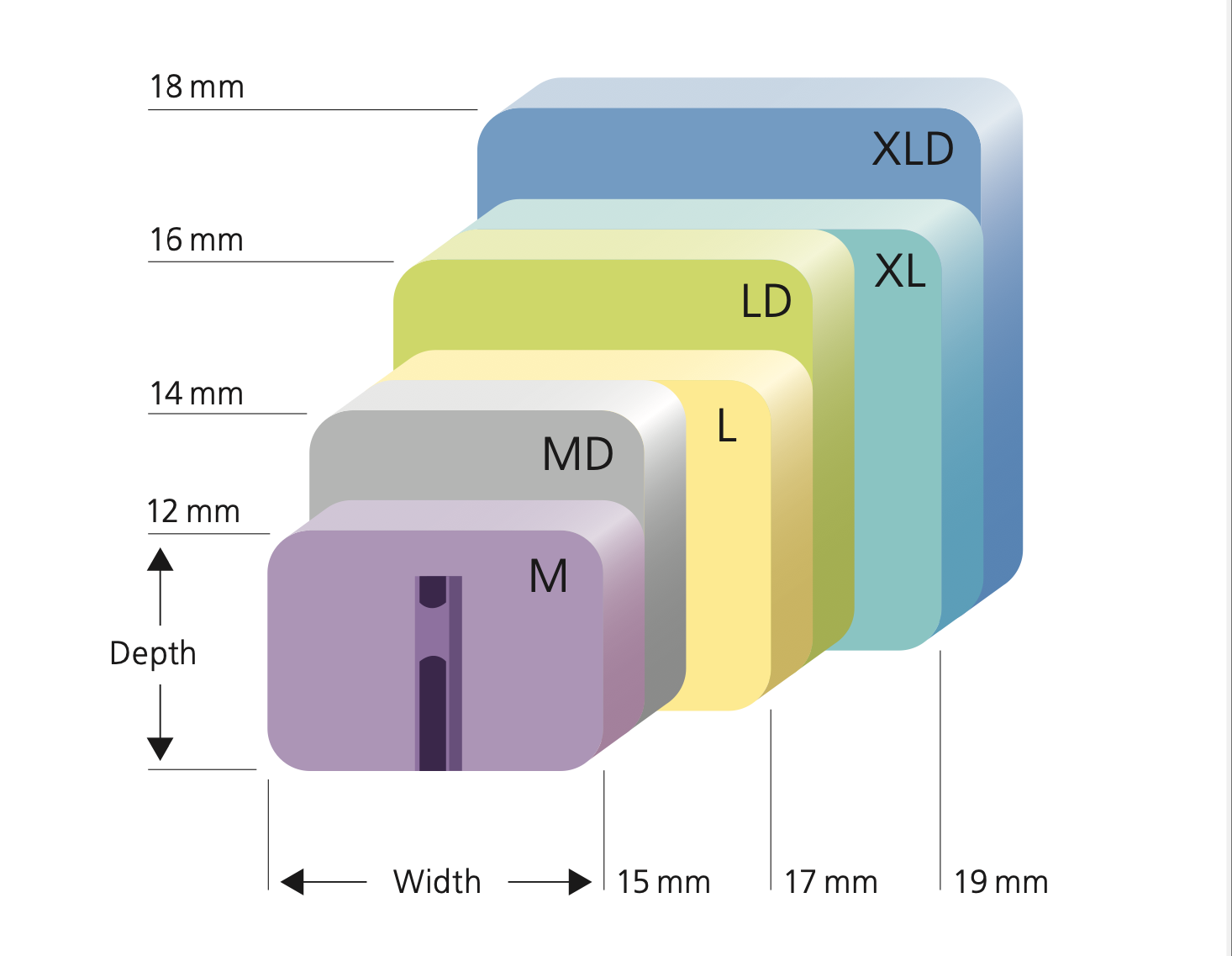
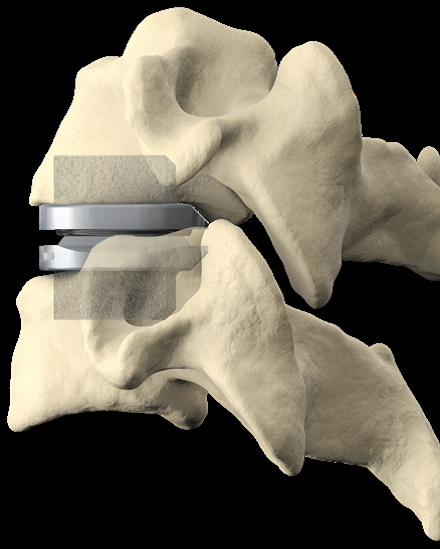
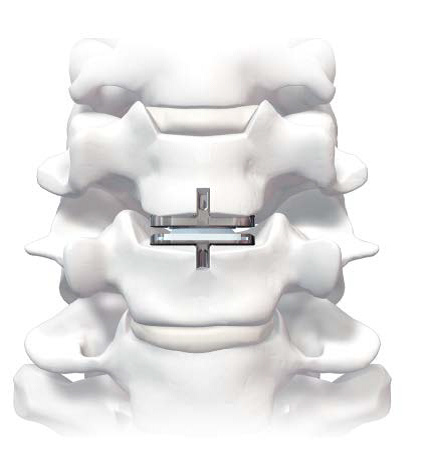
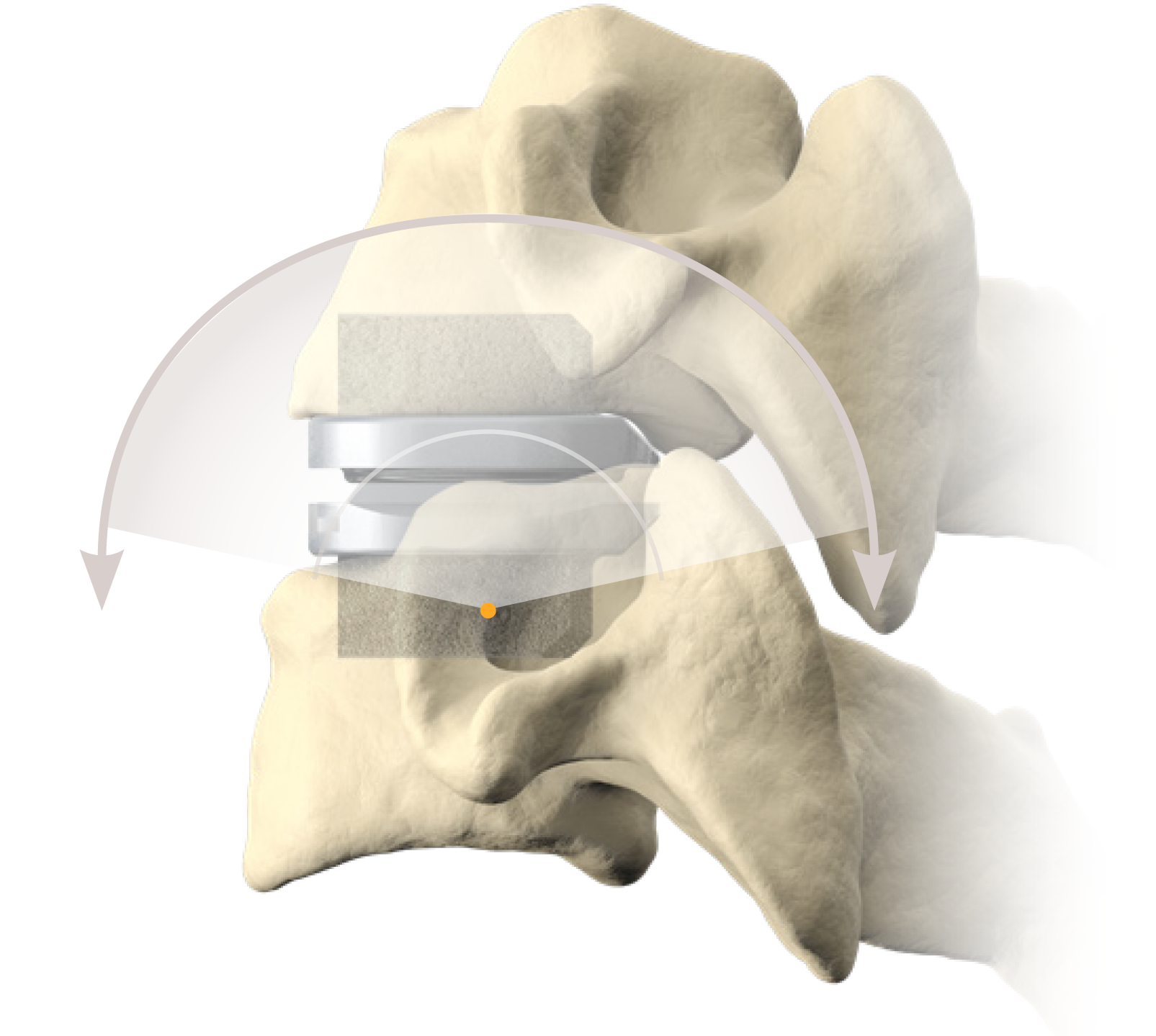
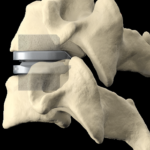
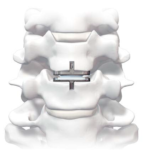
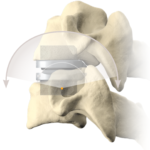
Cervical Disc Replacement surgery, a type of artificial disc replacement, can solve several causes of neck and upper back pain.
A cervical artificial disc replacement is a spine surgery procedure to remove a damaged vertebral disc and replace it with an artificial “joint.” There are many different types of artificial disc replacement. An artificial cervical disc is made of metals, and/or hard and soft plastics or polymers. Because a damaged cervical disc is often the source of pain, numbness, or tingling, once it is removed, pain is relieved. The artificial disc that replaces the natural disc, acts in much the same way as a natural vertebral disc. Once the patient heals after cervical artificial disc replacement surgery, the spine can rotate, bend, and flex without causing pain.

Types of Cervical Discs
There have been a number of discs released, each with varying degrees of success rates when stress-tested in clinical trials. Current FDA-approved discs include the Prestige-LP, Prestige-ST, prodisc® C, SECURE®-C and PCM artificial discs for the cervical spine.
Below is a topline overview of the FDA-approved alternatives currently available.
Approved & In Use
Prestige®-LP Cervical Disc
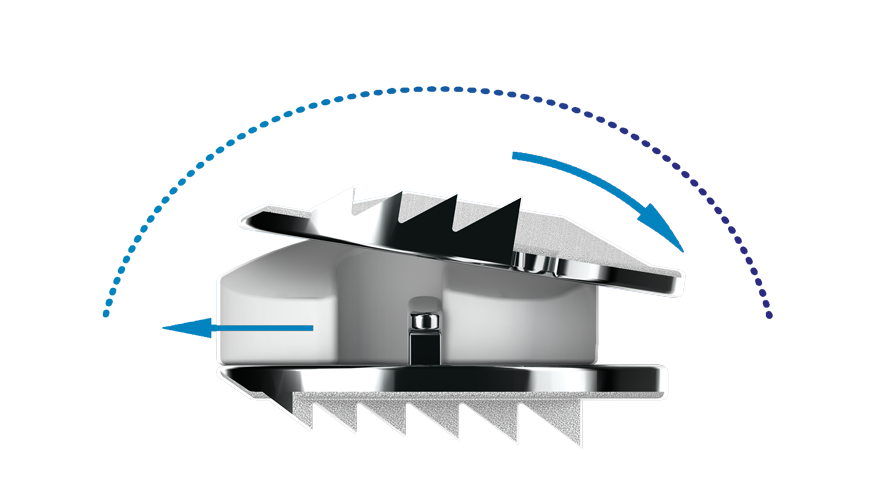
This low profile two-level disc, largely used with the military, is designed to preserve motion at the operated disc level, with the intention of relieving symptoms of a nerve root or spinal cord compression caused by a damaged disc. It consists of two pieces of metal inserted into the affected disc space of the neck after removing a diseased disc, to act like a pivoting joint, and is screwed in to reduce healing time.
SECURE®-C Cervical Disc
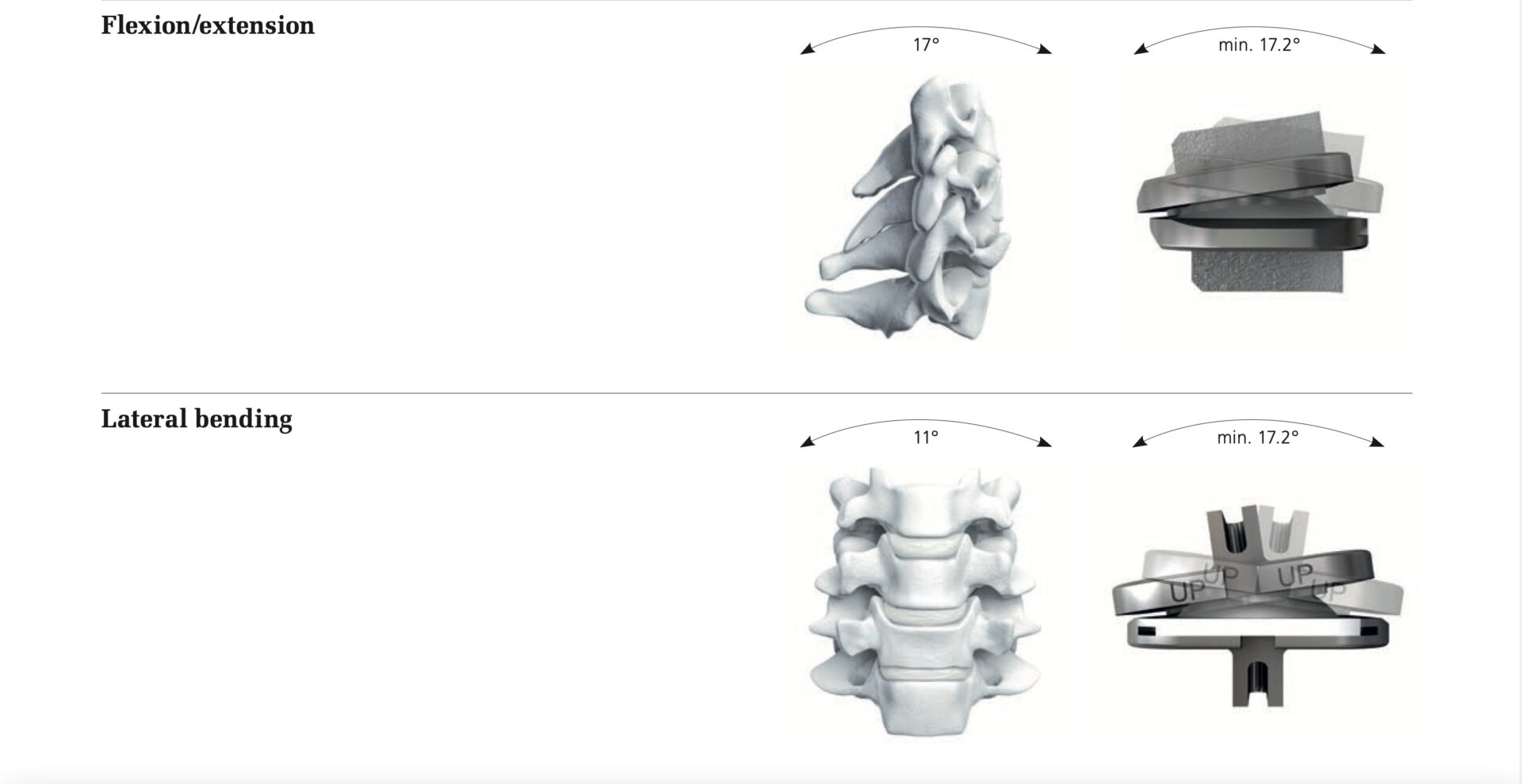
The SECURE®-C Cervical Artificial Disc was originally released with limited sizes, but has since been expanded. Its goal is to restructure the curve of the neck. The design is intended to also allow unlimited axial rotation, yet is constrained by ligaments and posterior elements. Disc suggests that it permits up to ±15º motion in flexion-extension (nodding up and down) and up to ±10º motion in lateral bending (tilting the head from side to side).
Simplify Disc
Featuring a variable center of rotation, the Simplify Disc is available in three disc heights, including the lowest available 4mm height. FDA approved for use in September 2020, following a superiority success rate of 93% when compared to spinal fusion. With its three-piece design available in 12 size combinations to fit a broad range of patient anatomies, the Simplify Disc is considered game-changing.
Mobi-C® Cervical Disc
Approved for use at one and or two levels, the Mobi-C® Cervical Disc has a patented mobile core designed to encourage height restoration and to facilitate motion close to the natural movement of the cervical spine. It features two intact bony end plates, composed of cobalt chromium molybdenum alloy and coated with plasma sprayed titanium and hydroxyapatite, with a polyethylene mobile bearing insert. It’s ideal for two-level implantation and offers one step insertion, with no additional operative steps such as bone chiseling or invasive keels or screws required.
prodisc® C Cervical Disc
Granted premarket approval in 2007, the prodisc® C is the ‘workhorse’ of artificial discs, designed to maintain the physiologic range of motion in the spine at one level from C3 to C7. Composed of three components - two cobalt chrome alloy endplates and an ultra-high molecular weight polyethylene inlay - it’s the first to be FDA-approved for cervical use. As one of the most widely-used discs, the prodisc® C is available in six different sizes and can be easily configured based on patient anatomy.
prodisc® C Cervical Disc
prodisc® C Vivo
Composed of four components to maximize restoration of motion, the prodisc® C Vivo has been designed to replace a diseased and/or degenerated intervertebral disc in patients with symptomatic cervical disc disease (SCDD). Composed of four components utilizing the tried and tested ball and socket design, the prodisc® C Vivo resists shear forces, allows for flexion, extension, rotation and lateral bending, with secure teeth fixation providing high level stability.
prodisc® C SK
Similar to the prodisc® C Vivo, the prodisc® C SK is also low profile, allowing for multilevel application (“stacking” of the discs at adjacent spinal levels), which is necessary for many patients who have more than one damaged disc in their neck that requires replacement. Rather than secure teeth fixation to hold the device in place, the prodisc® C SK incorporates low profile “keels” for fixation.
prodisc® C Nova
The prodisc C Nova implant has been designed to maintain the physiologic range of motion in the spine. The implant was developed using the clinically proven ball and socket concept used in joint replacement implants for over 40 years. The prodisc C Nova implant is composed of four components—two titanium alloy (TAN) endplates, a cobalt chrome alloy (CoCrMo) calotte insert, and an ultra-high molecular weight polyethylene (UHMWPE) inlay—and is inserted into the vertebral bodies en-bloc.
Approved & Discontinued
The following cervical discs have been discontinued.
PCM® Cervical Disc
The PCM® Artificial Disc is generally advised for skeletally mature patients for a reconstruction of a degenerative cervical disc at one level from C3-C4 and C6-C7. It’s a two-piece articulating device composed of two alloy metal end plates, with an ultra-high molecular weight polyethylene spacer fixed to the caudal end plate. A very mobile disc, while it was FDA-approved next to a fusion, it is now discontinued.
Prestige-ST Cervical Disc
The Prestige-ST was the first Medtronic disc, secured with screws to allow full combat within 14 days. The surface of the disc is grainy, allowing existing bone to regenerate and integrate into it. It’s held in place by four bone screws and two lock screws, utilizing a patented ball-and-trough design which allows for motion to be preserved. Made from cobalt chrome and nickel, the Prestige-ST was unsuitable for patients with allergies to metal, and has since been replaced with the Prestige®-LP Cervical Disc.
BRYAN® Cervical Disc
First reported to be used in 2002, the now obsolete BRYAN® consisted of two titanium alloy “shells” surrounding a saline-filled (prior to implantation) polycarbonate urethane nucleus that added compression to the disc. The bone-contacting edges of each shell were coated with sintered titanium to provide for bony ingrowth. Originally considered the best device to preserve motion, achieving similar results to that of a spinal fusion. While placement did prove to yield favorable clinical outcomes, the BRYAN® cervical disc also reported various issues related to the operative approach, decompression process, loosening of the device and subsidence of the implant, as well as postoperative kyphosis.
M6-C
The M6-C is an artificial disc replacement device that mimics the natural movement of an anatomical disc, while also allowing for compressibility via a unique viscoelastic core. Since FDA-approval in February 2019, the M6-C has established a market-leading position outside the United States, with over 54,000 devices implanted globally.
FDA Trials - Under Investigation
The following discs are currently under investigation. Patients can review eligibility criteria to see if they may be possible candidates for clinical trials. If you are interested in signing up for a trial please contact our office.
Synergy Disc™
The Synergy Disc™ is considered revolutionary in its design and functionality to treat cervical degenerative disc disease in patients who are unresponsive to conservative management. Available in three footprint sizes, two heights and two corrective lordotic angles, it’s the first disc that addresses patients with normal cervical lordosis and preoperative deformity. It offers a semi-constrained design with controlled motion stops, optimized for multi-level stability, to restore balance and mimic natural kinesthetic movement for enhanced motion preservation.
M6-C™
The Level 2 M6-C™ disc is considered ‘next generation’ in its ability to mimic the natural structure and movement of the spine, including forward, backwards, up, down, side to side and left to right rotations. Its innovative design composes two titanium endplates that anchor the disc to neighboring bone, as well as a shock-absorbing nucleus and fiber annulus that combine to replicate the cushioning effect and controlled range of movement of the natural disc, potentially minimizing stress to adjacent discs.
Off-Market European Devices
CP-ESP®
The CP-ESP® was developed for the cervical spine after a decade of success with its lumbar spine counterpart, the LP-ESP® lumbar disc. It employs innovative technology to mimic the natural movement of the cervical spine to six degrees, ensuring enhanced mobility and shock absorption to take pressure off the adjacent discs. It’s composed of two hydroxyapatite-coated titanium endplates with elastic nucleus (inner core) and elastic annulus (outer core).
Baguera®C
With an anatomical design composed of Diamond-Like-Carbon (DLC) coated titanium end plates and a guided mobile PE nucleus, the Baguera®C delivers reduced MRI artifact and better postoperative control. It allows six degrees of freedom and improved stress distribution, due to significantly lower contact pressure distribution on polyethylene central core.
Top Doctors Directory
Connecting Patients with top doctors in ADR and Arthroplasty. Search Top Doctors by name, city, and specialty.
A Holistic Approach
It’s rare to find a team who approach health the way we do. We take a holistic approach to your treatment, identifying ‘cause and effect’ to help ensure continued movement, flexibility and spinal restoration beyond surgical procedures.

“I'm sure you always hear how much you have made a difference in the quality of living after helping so many people, so you are going to hear it one more time from me. Thank you for everything and for being so sweet, patient and giving of yourself!”
We’ve changed the lives of thousands of people, including your favorite sports heroes and Hollywood stars, helping them to move better, every day.
“I'm sure you always hear how much you have made a difference in the quality of living after helping so many people, so you are going to hear it one more time from me. Thank you for everything and for being so sweet, patient and giving of yourself!”